Background: Renal impairment (RI) is one of the most common clinical presentations in patients with multiple myeloma (MM) and contributes to its morbidity and mortality. In consistent with this, renal injury is an important factor considered high risk for MM in recently updated NCCN clinical practice guideline. It has been demonstrated that RI is reversible in about half the patients with MM and reversal of renal dysfunction significantly affects the prognosis of MM (Blood Cancer J. 2014 Aug 1;4(8):e235). Because reversibility of RI was a more important prognostic factor than response to anti-myeloma therapy in patients with MM with RI (Clin Lymphoma Myeloma Leuk. 2022 Aug;22(8):626-634; Eur J Haematol. 2000 Sep;65(3):175-81), identification of accurate non-invasive predictive markers for differentiating reversible / irreversible RI in MM patients treated with anti-myeloma therapy is undoubtedly needed.
Methods: We conducted a retrospective study to evaluate the predicting value of serum erythropoietin (EPO) to albumin ratio (EPO/albumin) of MM patients treated with anti-myeloma therapy for reversibility of RI and overall survival (OS). RI was defined as an estimated glomerular filtration rate (eGFR) that was measured before treatment ≦50 ml/min per 1.73 m 2 by the simplified Modification of Diet in Renal Disease formula, and the definition of the reversibility of RI were also based on eGFR measurements, as previous publications described (J Clin Oncol. 2010 Nov 20;28(33):4976-84). Renal complete response (CRrenal) was defined as a sustained improvement from an eGFR baseline value of lower than 50 to ≥60 mL/min/1.73 m 2. Partial renal response (PRrenal) was defined as a sustained recovery of eGFR at the time of diagnosis of less than 15 to 30‐59 mL/min/1.73 m 2; when achieved CRrenal or PRrenal, the MM patients with RI is defined as the renal responder group, otherwise, the MM patients with RI is defined as the renal non‐responder group. Total 52 newly diagnosed MM patients with RI were analyzed in this study, 27 cases in the renal responder group and 25 cases in the renal non-responder group. Bortezomib with high-dose dexamethasone were used as the treatment of choice for such patients. Serum EPO levels were determined using chemiluminescence immunoassay. In our research, mean ± standard deviation (SD) was used to describe the normally distributed variables data while median with interquartile range (IQR) was used to describe the nonparametric variables data. P<0.05 was taken to indicate significance in all analyses.
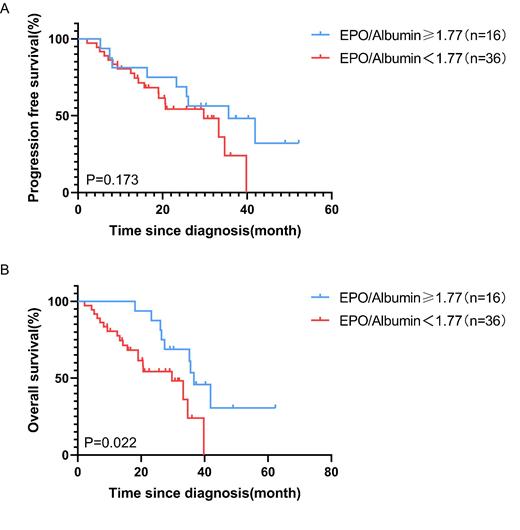
Results: There were significant differences in serum EPO and albumin, hemoglobin and RDW between the renal responder group and the non-responder group. The renal responder group had higher EPO [median: 44.06 (IQR: 160.96) vs 13.58(26.49)] and RDW [median:53(IQR:15) vs 48(9)], lower serum albumin (mean:30.21±SD: 7.78 vs 35.79±6.01) and hemoglobin. (mean:66.56±SD:16.42 vs 78.76±19.79), compared with the non-responder group. Of note, there were no significant differences in serum β2-Microglobulin, creatinine and eGFR at baseline between the two groups. As expected, serum EPO was negatively correlated with serum albumin (r = -0.257, P = 0.033). Likewise, EPO/albumin was negatively correlated with albumin (r = -0.42, P = 0.002); In contrast, EPO/albumin was positively correlated with serum EPO (r = 0.98, P < 0.001). Importantly, our study showed that EPO/albumin were independent predictor of the reversal of RI in MM patients. The optimal cutoff for EPO/albumin was determined via ROC analysis and was determined to be 1.77 U/g. The area under the curve was 0.784. According to the optimal cutoff value, low and high EPO/albumin groups were defined. The data confirmed that the group (n=36) with low EPO/albumin in the serum had shorter OS in MM with RI, compared with the group (n=16) with high EPO/albumin.
Conclusions: Our findings demonstrated that low EPO/albumin in the serum predicts less reversibility of RI with poorer prognosis in MM, it is a reliable biomarker for predicting reversibility of RI in MM patients before anti-myeloma therapy.
Figure 1
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal